Glenmark
- All
- News
- Videos
-

Cipla, Glenmark Recall Asthma Drugs Made In Indore From US Market
- Sunday May 5, 2024
- India News | Press Trust of India
Drug makers Cipla and Glenmark are recalling products from the American market due to manufacturing issues, according to the US health regulator.
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharma To Sell 75% Stake In Life Sciences To Nirma For $680 Million
- Thursday September 21, 2023
- India News | Reuters
Drugmaker Glenmark Pharmaceuticals said on Thursday it would sell a 75% stake in unit Glenmark Life Sciences to detergent maker Nirma for 56.52 billion rupees ($679.84 million).
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharmaceuticals Recalls 1,200 Bottles Of "Subpotent" Hypertension Drug In US
- Monday August 28, 2023
- India News | Press Trust of India
Glenmark Pharmaceuticals is recalling 1,200 bottles of a generic drug, used to treat high blood pressure, in the American market due to a manufacturing issue, according to the US Food and Drug Administration (USFDA).
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharmaceuticals Gets Final US Approval For Lacosamide Tablet
- Monday March 21, 2022
- India News | Press Trust of India
Homegrown pharma major Glenmark Pharmaceuticals Ltd on Monday said it has received final approval from the US health regulator for its generic Lacosamide tablet, indicated for prevention and control of seizures.
-
 www.ndtv.com
www.ndtv.com
-

5-Point Guide To The New Nasal Spray For Covid Cleared By India
- Wednesday February 9, 2022
- India News | Reported by Vishnu Som
A nasal spray to treat adult COVID-19 patients has been launched in India after Mumbai-based developers Glenmark Pharmaceuticals received marketing clearances from the Drugs Controller General of India as part of an accelerated approval process.
-
 www.ndtv.com
www.ndtv.com
-

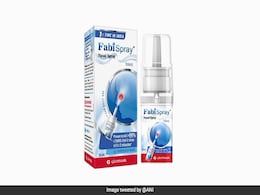
First Nasal Spray For Treating Adult Covid Patients Launched In India
- Wednesday February 9, 2022
- India News | Asian News International
Mumbai based innovation-driven global pharma company Glenmark has launched Nitric Oxide Nasal Spray (FabiSpray) in India, in partnership with SaNOtize, for treatment of adult patients suffering from COVID-19.
-
 www.ndtv.com
www.ndtv.com
-

Central Drug Agency Seeks Clarification From Glenmark On Its COVID-19 Drug
- Monday July 20, 2020
- India News | ANI
The Drugs Controller General of India (DCGI) has asked for a clarification from Glenmark Pharmaceuticals regarding its alleged "false claims" on the utilisation of anti-viral FabiFlu (favipiravir) on COVID-19 patients with comorbidities.
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Launches COVID-19 Drug Favipiravir At Rs 103 Per Tablet
- Sunday June 21, 2020
- India News | Press Trust of India
As Glenmark Pharmaceuticals launched antiviral drug Favipiravir for the treatment of mild to moderate COVID-19 cases, medical experts on Saturday cautioned against seeing it as a "magic bullet" to treat the deadly virus but said it will be helpful as it can be orally administered and reduce viral load.
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharma Receives 7 Observations From US Regulator On Baddi Unit, Shares Fall
- Tuesday November 28, 2017
- Business | NDTV Profit Team
Glenmark Pharmaceuticals said the Baddi unit contributes approximately 10 per cent of the revenue of US sales.
-
 www.ndtv.com/business
www.ndtv.com/business
-

High Court To Resume Hearing On Petitions Against Centre's Ban On Drugs
- Monday March 21, 2016
- Delhi News | Press Trust of India
Delhi High Court is likely to resume today the hearing on petitions by over 30 pharmaceutical majors, including Pfizer, Glenmark, Procter and Gamble (P&G) and Cipla, challenging the government's decision to ban sale of some of their fixed dose combination medicines.
-
 www.ndtv.com
www.ndtv.com
-

Delhi High Court Restrains Glenmark from Business of Anti-Diabetes Drugs
- Wednesday October 7, 2015
- India News | Press Trust of India
In a relief for US drug major Merck Sharp and Dohme (MSD), Delhi High Court today restrained Indian firm Glenmark Pharmaceuticals from manufacturing and selling its anti-diabetes drugs Zita and Zita-Met, saying it has infringed patent of the American company.
-
 www.ndtv.com
www.ndtv.com
-

Cipla, Glenmark Recall Asthma Drugs Made In Indore From US Market
- Sunday May 5, 2024
- India News | Press Trust of India
Drug makers Cipla and Glenmark are recalling products from the American market due to manufacturing issues, according to the US health regulator.
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharma To Sell 75% Stake In Life Sciences To Nirma For $680 Million
- Thursday September 21, 2023
- India News | Reuters
Drugmaker Glenmark Pharmaceuticals said on Thursday it would sell a 75% stake in unit Glenmark Life Sciences to detergent maker Nirma for 56.52 billion rupees ($679.84 million).
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharmaceuticals Recalls 1,200 Bottles Of "Subpotent" Hypertension Drug In US
- Monday August 28, 2023
- India News | Press Trust of India
Glenmark Pharmaceuticals is recalling 1,200 bottles of a generic drug, used to treat high blood pressure, in the American market due to a manufacturing issue, according to the US Food and Drug Administration (USFDA).
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharmaceuticals Gets Final US Approval For Lacosamide Tablet
- Monday March 21, 2022
- India News | Press Trust of India
Homegrown pharma major Glenmark Pharmaceuticals Ltd on Monday said it has received final approval from the US health regulator for its generic Lacosamide tablet, indicated for prevention and control of seizures.
-
 www.ndtv.com
www.ndtv.com
-

5-Point Guide To The New Nasal Spray For Covid Cleared By India
- Wednesday February 9, 2022
- India News | Reported by Vishnu Som
A nasal spray to treat adult COVID-19 patients has been launched in India after Mumbai-based developers Glenmark Pharmaceuticals received marketing clearances from the Drugs Controller General of India as part of an accelerated approval process.
-
 www.ndtv.com
www.ndtv.com
-

First Nasal Spray For Treating Adult Covid Patients Launched In India
- Wednesday February 9, 2022
- India News | Asian News International
Mumbai based innovation-driven global pharma company Glenmark has launched Nitric Oxide Nasal Spray (FabiSpray) in India, in partnership with SaNOtize, for treatment of adult patients suffering from COVID-19.
-
 www.ndtv.com
www.ndtv.com
-

Central Drug Agency Seeks Clarification From Glenmark On Its COVID-19 Drug
- Monday July 20, 2020
- India News | ANI
The Drugs Controller General of India (DCGI) has asked for a clarification from Glenmark Pharmaceuticals regarding its alleged "false claims" on the utilisation of anti-viral FabiFlu (favipiravir) on COVID-19 patients with comorbidities.
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Launches COVID-19 Drug Favipiravir At Rs 103 Per Tablet
- Sunday June 21, 2020
- India News | Press Trust of India
As Glenmark Pharmaceuticals launched antiviral drug Favipiravir for the treatment of mild to moderate COVID-19 cases, medical experts on Saturday cautioned against seeing it as a "magic bullet" to treat the deadly virus but said it will be helpful as it can be orally administered and reduce viral load.
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharma Receives 7 Observations From US Regulator On Baddi Unit, Shares Fall
- Tuesday November 28, 2017
- Business | NDTV Profit Team
Glenmark Pharmaceuticals said the Baddi unit contributes approximately 10 per cent of the revenue of US sales.
-
 www.ndtv.com/business
www.ndtv.com/business
-

High Court To Resume Hearing On Petitions Against Centre's Ban On Drugs
- Monday March 21, 2016
- Delhi News | Press Trust of India
Delhi High Court is likely to resume today the hearing on petitions by over 30 pharmaceutical majors, including Pfizer, Glenmark, Procter and Gamble (P&G) and Cipla, challenging the government's decision to ban sale of some of their fixed dose combination medicines.
-
 www.ndtv.com
www.ndtv.com
-

Delhi High Court Restrains Glenmark from Business of Anti-Diabetes Drugs
- Wednesday October 7, 2015
- India News | Press Trust of India
In a relief for US drug major Merck Sharp and Dohme (MSD), Delhi High Court today restrained Indian firm Glenmark Pharmaceuticals from manufacturing and selling its anti-diabetes drugs Zita and Zita-Met, saying it has infringed patent of the American company.
-
 www.ndtv.com
www.ndtv.com